Researchers from the Ye, Bohn, and Greene groups are busy exploring a cold new world crawling with polar hydroxyl radical (OH) molecules. The JILA experimentalists have already discovered how to cool OH to “lukewarm” temperatures of 30 mK. They’ve precisely measured four OH transition frequencies that will help physicists determine whether the fine structure constant has changed in the past 10 billion years.1 In a recent and productive collaboration between JILA experimentalists and theorists, the researchers have set ambitious goals: (1) figuring out how to cool large numbers of cold OH molecules to ultracold temperatures of less than 0.1 mK, (2) investigating and eventually controlling collisions of OH with other molecules, and (3) in the future, exploring ways to use the strong dipolar interaction in such molecules as molecular qubits in quantum computing.
The OH collaboration began with a theoretical analysis to determine whether OH could be sympathetically cooled with ultracold rubidium atoms in a magneto-optical trap (MOT). For such cooling to occur, there would need to be a high rate of collisions between the atoms and OH in which the OH molecules ended up in the same internal state after the collision as before, and colder. If the internal state changed, the molecules would actually get hotter and escape from the trap. After two years of painstaking analysis, research associate Manuel Lara, Fellow John Bohn, and their colleagues from the University of Durham (U.K.) and the Czech Technical University determined that the collision behavior of OH greatly diminished the prospects for sympathetic cooling. Bohn dubbed the proposed technique “simply pathetic cooling” and recommended keeping the nonspherical OH molecules out of magnetic traps altogether. A comparison of sympathetic and simply pathetic cooling is shown below.
Fortunately, all was not lost. The collaboration realized it had stumbled onto a very interesting collision process: In roughly one of every two collisions, the dipole moment of the OH would flip its direction; the rest of the time, the OH would maintain its original internal state. Every time an OH molecule flipped its dipole orientation, it would gain sufficient energy to fly out of a trap, making it easy to precisely measure the fraction of molecules changing state. Thus OH looked as if it might be ideal for studying cold molecule collisions — if the experimentalists could devise the right trap for it.
The more the scientists thought about it, the more fun and exciting it seemed to explore this new research avenue. They could still look for alternatives to sympathetic cooling for lowering the temperature of large numbers of OH molecules to µK levels, as the Ye and Greene groups are currently exploring. However, they could also study cold OH–OH collisions dominated by strong dipole-dipole interactions as well as other phenomena subject to similar long-range quantum influences. It occurred to them that since strong dipole-dipole interactions are sensitive to an external electric field, it might be possible to use tunable electric fields to control cold chemical reactions. Plus, Fellow Jun Ye’s NRC research associate Benjamin Lev realized that molecules like OH are viable candidates for encoding the qubits necessary for implementing quantum logic gates in quantum computers.
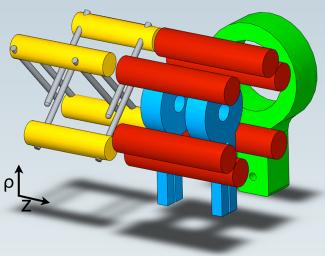
The next step in studying cold OH–OH collisions was the development of a new magneto-electrostatic trap in Ye’s lab by graduate student Brian Sawyer and Lev, with help from theorist Manuel Lara (Bohn’s research associate), former graduate student Eric Hudson, graduate student Ben Stuhl, Bohn, and Ye. It took Sawyer, Lev, Hudson, and Stuhl nine months to build the new apparatus. The hardest part was optimizing their decelerator to produce molecules moving slowly enough to trap. The new trap is shown above.
Trapping OH molecules began with making them in an electric discharge through water vapor followed by a supersonic expansion. This process created a molecular OH packet at a temperature of 1 K traveling at 490 m/s. The OH packet was filtered to 130 mK and slowed to 20m/s in an OH Stark decelerator developed in Ye’s lab several years ago by Jason Bochinski, Eric Hudson, and then NRC postdoc Heather Lewandowski, now an associate JILA Fellow. The end of the decelerator (yellow with grey electrodes in the illustration on the previous page) couples the now slowly moving cold molecule packets into the magnetic trap (blue), which is surrounded by electrodes (red) that create both uniform and nonuniform electric fields in the magnetic trap.
Once the OH molecules were magnetically trapped, Sawyer, Lev, and Stuhl measured how fast OH molecules fell out of the trap. They discovered that the applied electric field not only affected the number of molecules trapped, but also modified the trap itself, making it deeper, resulting in fewer molecular escapes. The electric field also changed the oscillation frequency of the OH molecules inside the trap. Interestingly, the magnetic and electric fields exerted comparable forces on the trapped molecules.
The best way to understand what was happening inside the new trap was to develop a better theory explaining the new trap’s operation. Because OH molecules possess both magnetic and electric dipoles whose directions can deviate from one another, just adding up the forces from the magnetic and electric fields didn’t work. The interaction of the magnetic and electric fields with the molecules had to be understood quantum mechanically. Sawyer did some of the field modeling, and he and Lev worked with Lara to figure out the detailed description of the molecules under the influence of both fields. Armed with new theoretical insights, the experimentalists were able to understand the OH dynamics they observed inside the trap, including collisions between OH and nitrogen (N2) molecules that occurred when the researchers let some air into the trap.
So what’s next? Sawyer, Lev, and Stuhl are already working on extending the lifetime of the new trap and increasing the number of OH molecules they can confine inside. Because they were able to observe collisions between OH and N2 molecules in the trap, the researchers are now evaluating ways to study collisions of OH with other polar molecules. In particular, they would like to see whether such collisions depend on the applied external electric field. If they can measure such a dependence, it would be the first time in cold matter physics research. It would also be a key first step in using electric fields to control cold molecule collisions and possibly even chemical reactions. - Julie Phillips


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.